





Gratton, Enrico
Laboratorio de Dinámica de fluorescencia. Universidad de California en Irvine. USA.
Lamy, María T.
Departamento de Física. Universidad de Sao Paulo. Sao Paulo. Brasil.
Grasa, María del Mar
Universidad Autónoma de Barcelona (UB). España.
Esteve, Montserrat
Universidad Autónoma de Barcelona (UB). España.
Mendoza, Aminta
Departamento de Física. Universidad Nacional de Colombia. Bogotá. Colombia.
Soulages, José Luis
Departamento de Bioquímica y Biología Molecular. Oklahoma. Universidad Estatal. USA.
Gennaro, Ana María
Departamento de Física. Universidad Nacional del Litoral. Santa Fé. Argentina.
Dataset of the construction and characterization of stable biological nanoparticles.
Gisonno, Romina A.; Tricerri, M. Alejandra; González, Marina C.; Garda, Horacio A.; Ramella, Nahuel A.; Díaz Ludovico, Ivo.
2020. Data in brief. Amsterdam: Elsevier Inc., vol. 33, ISSN 2352-3409
doi.org/10.1016/j.dib.2020.106536
Metodologías para la detección de SARS-CoV-2 y análisis de carga viral mediante RT-PCR cuantitativa.
Jaquenod De Giusti, Carolina; Montanaro, Mauro; Mencucci, Maria Victoria; Canzoneri, Romina; Orlowski, Alejandro; Santana, Marianela; Pereyra, Erica; Kraemer, Mauricio Horacio; Pedríni, Nicolás; González Baro, María; Vila Petroff, Martin; Aiello, Alejandro; Abba, Martin; Lavarías, Sabrina María Luisa; Moscoso, Verónica; Costantini, Noelian; Francini, Flavio; Garda, H.
2020. Innovación y desarrollo tecnológico y social (idts). La Plata: Universidad Nacional de La Plata. vol. 2, n° 2, p. 1-14
doi.org/10.24215/26838559e013
Triacylglycerol synthesis directed by glycerol-3-phosphate acyltransferases -3 and -4 is required for lipid droplet formation and the modulation of the inflammatory response during macrophage to foam cell transition.
Quiroga, Ivana Y.; Pellon-Maison, Magali; González, Marina C.; Coleman, Rosalind A.; González Baro, Maria R.
2020. Atherosclerosis. ELSEVIER IRELAND LTD. ISSN 0021-9150
doi.org/10.1016/j.atherosclerosis.2020.11.022
Potential Inhibitors of the Activity of the Cholesterol-Ester Transfer Protein.
Tárraga, Wilson Alberto; Garda, Horacio Alberto; Toledo, Juan Domingo; González, Marina Cecilia.
2019: Journal of computational biology. New York: Mary Ann Liebert, Inc., vol. 26, n° 12, p. 1458-1469.
doi.org/10.1089/cmb.2018.0227
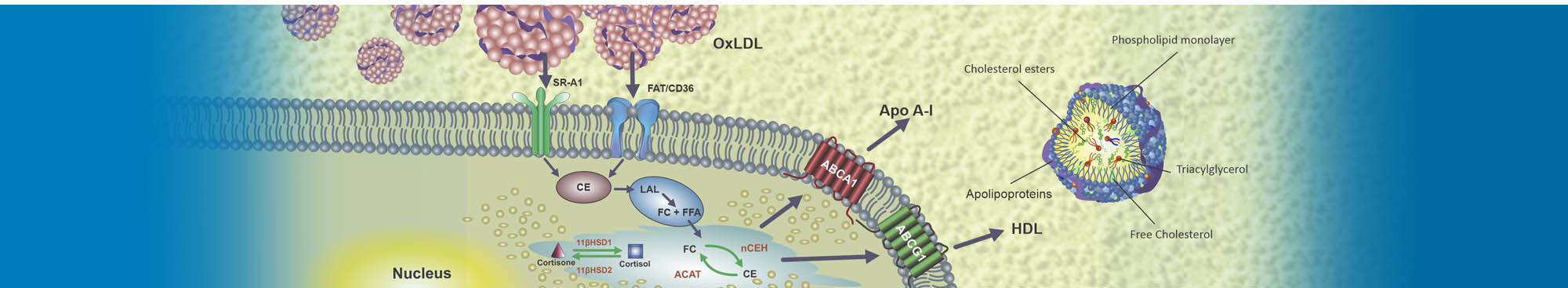
Role of cortisol in inflammationtriggered by macrophages exposition to modified LDL.
Toledo, J. D; Montserrat, E; Grasa, M. Del M; Ledda, A; Garda, H. A; Gulfo, J; Díaz, L. I; Ramella, N; Gonzalez, M. C.
2016. Data in brief-journal: Elsevier, vol. 8, p. 251-257
Data related to inflammation and cholesterol deposition triggered by macrophages exposition to modified LDL.
Toledo, J. D; Rafols, M; Grasa, M; Ledda, A; Garda, H. A; Gulfo, J; Diaz, L. I; Ramella, N; Gonzalez, M. C.
2016. Data in brief: Elsevier, vol. 8, p. 251-257
Decreased OxLDL uptake and cholesterol efflux in THP1cells elicited by cortisol and by cortisone through 11b-hydroxysteroid dehydrogenase type 1.
Ledda, A; Gonzalez. M; Gulfo, J; Díaz, L. I; Ramella, N; Toledo, J; Garda, H; Grasa, M; Montserrat, E.
2016. Atherosclerosis. Amsterdam: ELSEVIER IRELAND LTD, vol. 250, p. 84-94. ISSN 0021-9150
Aminolevulinic acid dendrimers in photodynamic treatment of cancer and atheromatous disease.
Rodriguez, L; Vallecorsa, P; Battah, S; Di Venosa, G; Calvo, G; Mamone, L; Saenz, D; Gonzalez, M. C; Batlle, A; Macrobert, A. J; Casas, A.
2015. Photochemical and Photobiological Sciences. CAMBRIDGE: ROYAL SOC CHEMISTRY. vol. 14, n° 9, p. 1617-1627. ISSN 1474-905X
Apolipoprotein A-I configuration and cell cholesterol efflux activity of discoidal lipoproteins depend on the reconstitution process.
Cuellar, L. A; Prieto, E; Cabaleiro, L; Garda, H. A.
2014. Biochimica et Biophysica acta-molecular and cell biology of lipids. Amsterdam: ELSEVIER SCIENCE BV,. vol. 1841, p. 180-189. ISSN 1388-1981
Apolipoprotein A-I Helsinki promotes intracellular acyl-CoA cholesterol acyltransferase (ACAT) protein accumulation.
Toledo, J. D; Garda, H. A; Cabaleiro, L. V; Cuellar, A; Pellón Maison, M; González Baró, M; Gonzalez M. C.
2013. Molecular and cellular biochemistry. New York: SPRINGER. vol. 377, n° 1-2, p. 197-205. ISSN 0300-8177
Apolipoproteína A-I y lipoproteínas de alta densidad: Estructura y rol en la homeostasis del colesterol celular.
Garda, H. A; Toledo, J. D; González, M. C; Prieto, E; Cuellar, L. A; Cabaleiro, L. V; Chirillano, L. A; Almeyra, C. M.
2013. Acta Bioquímica Clínica Latinoamericana. La Plata: Federacion Bioquímica Provincia Buenos Aires, vol. 47, n° 2, p. 327-341. ISSN 0325-2957
El Dr. Rodolfo R. Brennner y una de sus principales obras: El Instituto de Investigaciones Bioquímicas de La Plata (INIBIOLP).
Garda, H. A.
2013. La Plata. Acta Bioquímica Clínica Latinoamericana. Federacion Bioquímica Provincia Buenos Aires. vol. 47, n° 2, p. 249-266. ISSN 0325-2957
Characterization of a human apolipoprotein A-I construct expressed in a bacterial system.
Prieto, E. D; Ramella, N; Cuellar, L. A; Tricerri, M. A; Garda, H. A.
2012. Protein journal. Berlin: Springer, vol. 31, n° 8, p. 681-688
Effect of reconstituted discoidal high density lipoproteins on lipid mobilization in RAW 264.7 and CHOK1 cells.
Toledo, J. D; Cabaleiro, L. V; Garda, H. A; González, M. C.
2012. Journal of cellular biochemistry. New York: Wiley-Liss, Div John Wiley & Sons Inc. vol. 113, n° 4, p. 1208-1216
Membrane insertion topology of the central apolipoprotein A-I region. Fluorescence studies using single tryptophan mutants.
Prieto, E. D; Garda, H. A.
2011. Biochemistry 50, 466 - 79. Editorial: American Chemical Society.
Human Apolipoprotein A-I-Derived Amyloid: it’s Association with Atherosclerosis.
Ramella, N. A; Rimoldi, O. J; Prieto, E. D; Schinella, G. R; Sanchez, S. A; Jaureguiberry, M.S; Vela, M.E; Ferreira, S. T; Tricerri, M. A.
2011. PLoS One. 2011 ;6 (7): e22532. Epub 2011 Jul 19
Structure and stability of crustacean lipovitellin: Influence of lipid content and composition.
García, C. F; Cunningham, M; Soulages, J. L; Heras, H; Garda, H. A.
2010. Comparative biochemistry and physiology. part b, biochemistry & molecular biology. Elsevier Science Inc. vol. 155, n° 2, p. 126 - 131
Membrane Organization and Regulation of Cellular Cholesterol.
Jaureguiberry, M. S; Tricerri, M. A; Sanchez, S. A; Garda, H. A; Finarelli, G. S; Gonzalez, M. C; Rimoldi. O. J.
2010. Journal of membrane biology. New York: Springer. vol. 234, p. 183 - 194
The central type Y amphipathic ahelices of apolipoprotein AI are involved in the mobilization of intracellular cholesterol depots.
González, M. C; Toledo, J. D; Tricerri, M. A; Garda, H. A.
2008. Archives of biochemistry and biophysics. Amsterdam: Elsevier, vol. 473, n° 1, p. 34 - 41
Embryo lipoproteins and yolk lipovitellin consumption during embryogenesis in Macrobrachium borellii (Crustacea: Palaemonidae).
García, C. F; Cunningham, M; Garda, H. A; Heras, H.
2008. Comparative biochemistry and physiology. part b, biochemistry & molecular biology, vol. 151, p. 317 - 322
“Premio SAB al mejor trabajo”
III Congreso Iberoamericano de Biofísica y XXVI Reunión Anual de la Sociedad Argentina de Biofísica. Buenos Aires. Septiembre de 1997
Conformation of apolipoprotein A-I in reconstituted lipoprotein particles and particle-membrane interaction. Effect of size and cholesterol.
Tricerri, M. A; Córsico, B; Toledo, J. D; Garda, H. A; Brenner, R. R.
“Premio SAB al mejor trabajo”
XXXI Reunión Anual de SAB. Buenos Aires. Diciembre de 2002
Interacción del dominio central de apolipoproteína A-I con membranas.
Prieto, E. D; Toledo, J. D; Garda, H. A.
“Mención de honor”
IV Congresso de Biofísica do Cone-Sul XXIX Reunión Anual de SAB. Campinas, SP, Brazil. Agosto de 2000
Trabajo: Interacción de complejos lipoproteicos discoidales de apolipoproteína A-I con vesículas lipídicas estudiada con reactivos fotoactivables.
Córsico, B; Toledo, J. D; Garda, H. A.
“Mejor poster de ciencias básicas”
Otorgado en Jornadas de la Facultad de Ciencias Médicas. La Plata. Octubre de 2010. Correspondiente al trabajo:
Configuración de la apolipoproteína A-I ( LL5/2 en dHDL) fisiológicamente activa en la remoción de colesterol de células RAW.
Cuellar, L. A; Prieto, E. D; Cabaleiro, L. V; González, M. C; Garda, H. A.